Salt-in-Ionic-Liquid Electrolytes: Ion Network Formation and Negative Effective Charges of Alkali Metal Cations
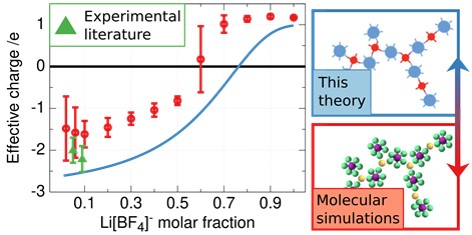
Salt-in-ionic liquid electrolytes have attracted significant attention as potential electrolytes for next generation batteries largely due to their safety enhancements over typical organic electrolytes and promising compatibility with energy-dense alkali-metal anodes.
Recent experimental and computational studies have shown that under certain conditions alkali cations can migrate in electric fields as if they carried a net negative effective charge at small mole fractions of alkali metal salt that reverts to the expected net positive values at large mole fractions. Using the molecular simulations we extract the parameters required by the developed theory, such as the functionality of the ions (number of associations that they can form), strength of associations, amongst other parameters. Overall, we find excellent qualitative agreement between our theory, parameterised by the simulations, and molecular simulations in terms of ionic cluster statistics and the effective charges of the alkali cations.
Link to the publication: https://pubs.acs.org/doi/10.1021/acs.jpcb.1c05546
Authors: Michael McEldrew; Zachary A. H. Goodwin; Nicola Molinari; Boris Kozinsky; Alexei A. Kornyshev; Martin Z. Bazant
