Structure and Migration Mechanisms of Oxygen Interstitial Defects in β-Ga₂O₃
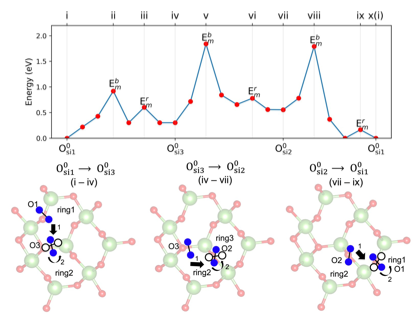
Gallium oxide (β-Ga₂O₃) has emerged as a promising material for next-generation electronic applications, including power devices and photodetectors. However, the presence of crystal defects, particularly oxygen interstitials (Oi), can significantly impact its performance. In this study, we systematically investigate the structures and diffusion mechanisms of interstitial oxygen in β-Ga₂O₃ across different charge states using non-local density functional theory. Our results provide a detailed map of the potential energy surface of Oi in β-Ga₂O₃, and demonstrate how these ions can migrate between different rings in the structure. The results indicate that Oi atoms can form O-O dimer configurations at three distinct oxygen sites in β-Ga₂O₃, acting as split interstitial defects in neutral (0) and positively charged (+1) states. However, at higher Fermi levels (above 1 eV from the conduction band minimum), additional electron trapping can break the O-O dimers, leading to oxygen interstitials in the negatively charged (-1 and -2) states. Furthermore, we analyze the migration mechanisms of O-O dimers and identify two primary processes: bond-breaking and dimer rotation between different structural rings in the lattice. These findings are expected to be of interest to the broader research community working on ultra-wide bandgap semiconductors.
Authors: Chaiyawat Kaewmeechai, Jack Strand, Alexander L. Shluger
