The role of copper in enhancing the performance of heteronuclear diatomic catalysts for the electrochemical CO2 conversion to C1 chemicals
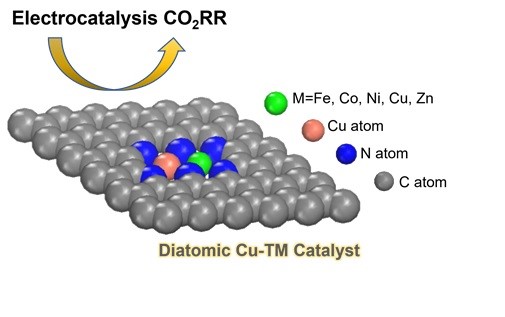
TYC members, Zhao and Di Tommaso (Queen Mary), and Crespo-Otero (UCL) have identified the role of copper (Cu) in diatomic catalysts in the electrochemical CO2 conversion to one-carbon (C1) value-added chemicals. Traditionally, Cu has been identified as the catalytic site for CO2 reduction. However, their work challenges this conventional understanding by introducing designs involving both single-atom and Cu-based bimetallic catalysts for CO2 reduction studies.
Utilising first principles calculations based on density functional theory and a computational hydrogen electrode model, the authors have unravelled the fundamental mechanisms at play, revealing that copper assumes an exceptional auxiliary role in the CO2 conversion process. Acting as a proficient promoter, Cu facilitates the activation and reduction of CO2 by collaborating with other non-precious metal species that are abundant in the Earth’s crust. This ground breaking insight opens new avenues for understanding and enhancing the efficiency of CO2 reduction processes through synergistic interactions between copper and other abundant metal constituents.
Authors: Qi Zhao, Rachel Crespo-Otero, and Devis Di Tommaso
